The Importance of pH Levels in Water Treatment
Water is a vital and main resource for all living organisms on Earth, and its quality is paramount. One critical aspect that often goes unnoticed but plays a significant role in water treatment is pH levels. pH of water, which stands for “potential of hydrogen,” is a measure of the acidity or alkalinity of a water solution. In the realm of water treatment, maintaining the right pH levels is paramount. This information will delve into the importance of pH levels in water treatment, exploring why they matter and how they impact various processes.
Understanding pH Levels
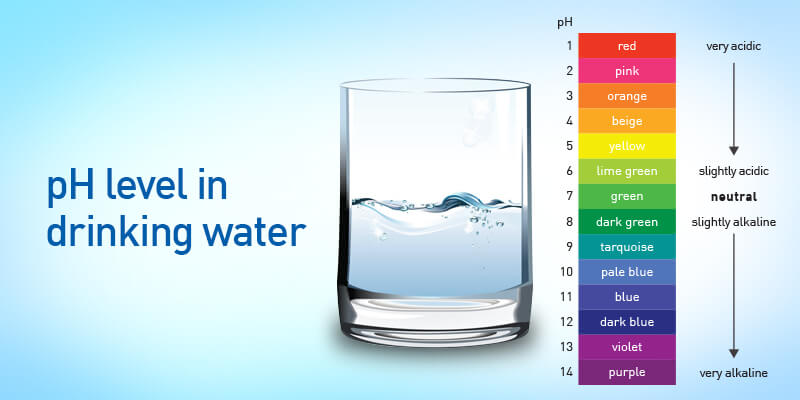
Before we dive deeper into the significance of pH levels in water treatment, let’s first understand what pH is and how it is measured. pH is measured from 0 to 14 scales, with seven considered neutral. Values below 7 indicate acidity, while values above 7 signify alkalinity. Water with a pH of 7 scale is neither acidic nor alkaline; it is neutral. A change in pH by one unit represents a tenfold change in acidity or alkalinity.
The Role of pH in Water
Now that we have a basic understanding of pH, let’s explore its Role in Water and why it is crucial in the treatment process.
- Chemical Reactions
pH levels influence various chemical reactions that occur during water treatment. For instance, adjusting the pH can aid in the coagulation and flocculation processes, essential for removing impurities and particles from Water.
- Disinfection
Maintaining the right pH level is vital for effective disinfection. Chlorination, a common method for disinfecting Water, is most effective when pH levels are within a specific range. Deviations from this range can reduce the disinfection efficiency.
- Corrosion Control
pH levels also play a significant role in controlling corrosion in water distribution systems. Water that is too acidic or alkaline can lead to the corrosion of the pipes and fixtures, potentially contaminating the Water.
pH Levels in Different Water Sources
Different water sources have varying pH levels naturally. It is essential to understand these variations to ensure proper treatment.
- Groundwater
Groundwater sources typically have a higher pH, making them slightly alkaline. Treating groundwater may require adjusting pH levels to meet regulatory standards.
- Surface Water
Surface water sources like rivers and lakes can have varying pH levels depending on weather and human activities. Treating surface water often involves pH adjustment to ensure safety.
- Wastewater
Wastewater, which includes industrial and domestic effluents, can have highly variable pH levels. Proper pH control is critical to treat and safely discharge wastewater into the environment.
Conclusion
In conclusion, pH levels are a fundamental aspect of water treatment. They influence chemical reactions, disinfection processes, and corrosion control, crucial for providing safe and clean water to communities. Maintaining the right pH levels in different water sources ensures efficient and effective treatment.
Frequently Asked Questions
- What is the ideal pH level for drinking Water?
The ideal and effective pH level for drinking Water is typically between 6.5 and 8.5. This range ensures that the Water is neither too acidic nor too alkaline for human consumption.
- How can I test the pH level of my tap water at home?
You can test the pH level of your tap water using pH test strips or a pH meter, which are readily available at most hardware stores or online retailers.
- Why is pH control essential in wastewater treatment?
pH control in wastewater treatment is essential to ensure the efficiency of the treatment process and prevent environmental harm. Extreme pH levels can interfere with the removal of contaminants and impact the safety of discharged Water.
- Can pH levels in water change naturally?
Yes, pH levels in Water can change naturally due to factors like geological conditions, rainfall, and the presence of minerals. Human activities, such as pollution, can also alter pH levels.
- What are the potential or main health risks of drinking Water with unbalanced pH levels?
Drinking Water with excessively high or low pH levels can lead to health issues. High acidity or alkalinity can cause gastrointestinal problems and affect the taste and odor of Water. It’s essential to maintain balanced pH levels for safe drinking water.